Recently, researchers from Hyundai Motor Co. found that sulfone-based electrolytes can effectively increase the capacity and reversible capacity retention of lithium-sulfur batteries. In the 2014 American Society of Automotive Engineers World Congress, Hyundai Motor Co. reported on the above new findings. Compared with ordinary electrolytes, the capacity of lithium-sulfur batteries can be effectively increased by using sulfone-based electrolytes. The capacity is increased by 52.1% to 715 mAh. Time/g; reversible capacity retention increased by 63.1% to 72.6%.
This article refers to the address: http://
As a new material battery with an energy density exceeding the lithium-ion battery, the lithium-sulfur battery has a larger battery capacity, and the electric vehicle with the battery will have a farther electric range. The theoretical energy density of lithium-sulfur battery systems has reached 2,600 watt-hours/kg, but its low reversible capacity retention rate is a well-known problem. At the same time, lithium-sulfur batteries also have problems in that the polysulfide compound (PS) is dissolved in the electrolyte and solid lithium sulfide and other insoluble precipitates are generated on the cathode during discharge.
Hyundai Motor Company researcher Shin et al. said: "The reaction mechanism of lithium-sulfur battery is that the negative metal lithium loses electrons into lithium ions during discharge, and the positive sulfur reacts with lithium ions and electrons to form polysulfide (multi-sulfide PS is contained). A sulfur ion compound, wherein the specific reaction process is S8→Li2S8→Li2S6→Li2S4→Li2S), and the potential difference between the positive electrode and the negative electrode is the discharge voltage provided by the lithium sulfur battery. Under the action of the applied voltage, the positive electrode of the lithium sulfur battery The negative electrode reaction proceeds in the reverse direction, which is the charging process, and a reversible reaction occurs during the charging process. Li2S6 and Li2S4 can be dissolved in the electrolyte during the reaction of polysulfide. The sulfur utilization rate of the lithium-sulfur battery is improved to improve the reversible recycling of the battery. In terms of rate, polysulfide plays a crucial role.
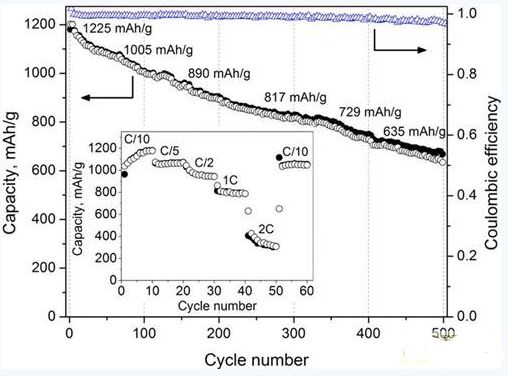
Ether-type solvents are considered to be the best electrolyte choice for lithium-sulfur batteries due to their good polysulfide solubility and high chemical stability. In addition, the dissolved polysulfide initiates a redox reaction, which lowers the cell coulombic efficiency and shortens the reversible cycle retention rate, resulting in self-discharge. Therefore, the main purpose of this research and development work is to develop a new electrolyte to reduce the redox reaction and improve the reversible cycle retention rate of the battery. â€
In the research process of Hyundai Motor Company, the researchers used five groups of monoether ether electrolytes (dimethyl ether DME, diethylene glycol dimethyl ether DEGDME, triethylene glycol Triglyme, triglyme dimethyl ether TEGDME and two Oxygen hexacyclic DIOX), a group of binary ether electrolytes (triethylene glycol dimethyl ether TEGDME and dioxane DIOX mixture) and three groups of ternary ether electrolytes (mixing ratio of 1:1:1, 1, respectively) : 1:2 and 1:1:3 triethylene glycol dimethyl ether TEGDME: dioxane DIOX: sulfolane Sulfolane mixed electrolyte) were compared respectively.
The lithium-sulfur battery used by Hyundai Motor Company's researchers used a sulfur cathode and a lithium metal foil anode, and a polyethylene diaphragm was used between the two electrodes. The lithium-sulfur battery electrochemical experiment was carried out at room temperature of 20 ° C and the operating voltage was controlled between 1.5 volts and 2.65 volts.
In the monoether ether electrolyte experiment, the dimethyl ether DME electrolyte system has the highest energy density, reaching 878 mAh / gram; the diethylene glycol dimethyl ether DEGDME electrolyte system energy density is second, also reached 857 mAh Time / gram. However, the dimethyl ether DME electrolyte system showed a very significant battery capacity decay after the sixth working cycle; while the diethylene glycol dimethyl ether DEGDME electrolyte system showed a very obvious battery after the second working cycle. Capacity attenuation. The dioxane DIOX electrolyte system achieved an energy density of 1040 mAh/g during the first working cycle, while the energy density rapidly dropped to 640 mAh/g during the 12th duty cycle. The dioxane DIOX electrolyte system has a very high initial energy density, however, its energy density also shows a very significant battery capacity decay after the 12th duty cycle. The triethylene glycol dimethyl ether TEGDME electrolyte system has a low initial energy density of only 200 mAh/g, but no significant battery capacity degradation occurs during the subsequent duty cycle.
In the binary ether electrolyte experiment, the experimenter obtained the binary ether electrolyte by mixing 1:1 ethylene glycol dimethyl ether TEGDME and dioxane DIOX. The purpose of this experiment was to take advantage of the good reversible cycle retention of triethylene glycol dimethyl ether TEGDME and the high energy density of dioxane DIOX. It was found by experiments that the initial energy density of the binary ether electrolyte system reached 1057 mAh/g, and the energy density was 470 mAh/g after 20 working cycles. The binary ether electrolyte exhibits a good reversible cycle retention ratio as compared with the monoether ether electrolyte. However, the binary ether electrolyte system still has significant battery capacity decay after the first working cycle, and after 20 working cycles, the binary ether electrolyte system has a low reversible cycle retention rate of only 44.5. %.
In the binary ether electrolyte experiment, the experimenter also added a glass membrane filter between the two electrodes of the lithium-sulfur battery, in order to suppress the high impedance around the electrode of the lithium-sulfur battery. The glass transmembrane filter can attract electrolytes, so the possibility of electrolyte deficiency around the electrodes can be effectively reduced by adding a glass transmembrane filter. By using a glass-exchange membrane filter, the initial energy density of the binary ether electrolyte system is reduced, and the reversible cycle retention rate is improved, and the energy density can reach 605 mAh/g after 20 working cycles.
According to the chemical analysis of researchers at Hyundai Motor Co., the sulfone-based electrolyte can form a protective film on the surface of the anode of the lithium-sulfur battery and can reduce the precipitation of polysulfide by blocking the reaction between the lithium metal anode and the polysulfide. In addition, ordinary electrolytes may have cracks on the surface of the battery electrode during the reaction, and the protective film can effectively reduce the occurrence of cracks on the surface of the electrode.
In the ternary ether electrolyte experiment, Hyundai Motor's research team used sulfolane Sulfolane as its lithium-sulfur battery electrolyte. Different ratios of electrolyte solutions were obtained by mixing different doses of sulfolane Sulfolane with triethylene glycol dimethyl ether TEGDME and dioxane DIOX. The experimental results show that the 1:1:2 ratio of triethylene glycol dimethyl ether TEGDME, dioxane DIOX, sulfolane Sulfolane mixed electrolyte has the best reversible cycle retention rate, and the battery capacity has reached 715 mAh /å…‹; and 1:1:1 ratio of triethylene glycol dimethyl ether TEGDME, dioxane DIOX, sulfolane Sulfolane mixed electrolyte followed by a battery capacity of 674 mAh / gram, reversible cycle retention rate of 68 %; 1:1:3 ratio of triethylene glycol dimethyl ether TEGDME, dioxane DIOX, sulfolane Sulfolane mixed electrolyte in all aspects of performance is the worst. In addition, in the ternary ether electrolyte experiment, the surface crack phenomenon of the anode of the lithium sulfur battery was significantly reduced.
We make OBD connector with terminal by ourselves, soldering type and crimping type are both available. Also 12V and 24V type. OBD1, OB2, J1939, J1708, J1962, etc. Also molded by different type, straight type or right-angle type. The OBD connector cables used for Audi, Honda, Toyota, BWM, etc. We have wide range of materials source , also we can support customers to make a customized one to replace the original ones.
OBD Connectors,Sae J1708 Connector,Sae J1939 Connector,OBD2 Diagnostic Connectors,Diagnostic Connector,Deutsch Diagnostic Connector
ETOP WIREHARNESS LIMITED , https://www.etopwireharness.com